Our research
How FOL005 works
A clinically validated peptide designed to stimulate hair growth
FOL005 is a unique and patented peptide designed for hair growth stimulation through topical administration with a unique proprietary formulation for application once daily (Figure 4A).
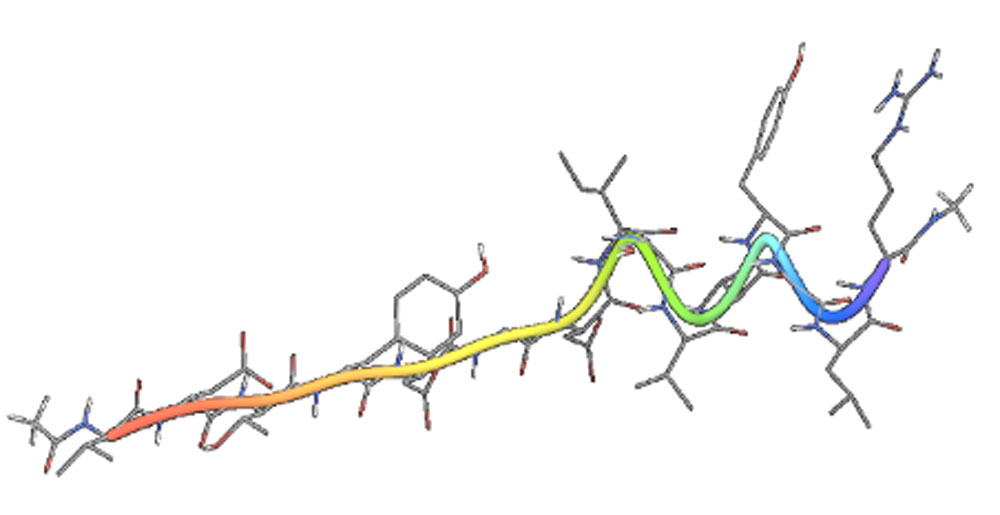
The small peptide is based on a modified part of the endogenous human structural protein osteopontin, a glycoprotein expressed by many tissues, among these also the hair follicle, bone and involved in inflammatory processes. FOL005 is unique and covered by patents until 2040.
The sequence is based on natural amino acids and was modified to optimize the hair growth potential and in order to ensure stability of the product for several years at room temperature.
Clinical studies including more than 300 people with androgenetic alopecia demonstrated that FOL005 resulted in remarkable growth of new hairs for those who had a hair density less than 255 hairs per square centimeter after once daily application for only 4 months. FOL005 applied once daily induces hair growth after 4 months comparable to minoxidil applied twice daily for six months.
Topical formulation with unique properties
The proprietary topical formulation is a non-running gel which is easy to apply. The formulation keeps the FOL005 stable for several years at room temperature. Furthermore, the formulation is designed to ensure good skin penetration and distribution of FOL005 in the epidermis and the hair follicles.
The background of Follicum AB and FOL005
In 2004 the founders of Follicum, among others Prof. Anna Hultgårdh Nilsson discovered, in connection with research on arteriosclerosis, that a modified protein increased in preclinical models. The modified protein FOL005 was derived from the human glycoprotein osteopontin (OPN). OPN is an extracellular matrix glycoprotein with diverse immunomodulatory functions that has been associated with inflammation and fibrosis, but some publications also report OPN to be present in hair follicles in a hair cycle dependent manner. FOL005 has demonstrated proof-of-concept in a Phase 2a clinical study and has been tested in more than 300 persons suffering from hairloss.
Hairloss effects almost half of men and women
In men androgenetic alopecia is a genetically predetermined disorder due to an excessive response to androgens. However, the commonality between men and women is likely due to the muscle supporting the hair follicle (the arrector pili muscle), where a loss of attachment between the muscle and hair follicle bulge is associated with irreversible or partially reversible hair loss.
A key driver for this is due to diminished blood flow to the muscle. Androgenetic alopecia develops slowly over time and it is caused by the hair follicle becoming smaller and, in the end, it is ultimately inactive and is not able to grow new hair, a process called miniaturization. Each hair originates in a hair follicle, and a cyclic process known as the hair growth cycle, that consists of four phases:
- The growth (anagen) phase, (2 to 7 years),
- The transition (catagen) phase, (2 weeks),
- The resting (telogen) phase where old hair is removed, (12 weeks), and
- The release (exogen) phase, which is the release phase of the telogen hair.
Miniaturization occurs at some point between the late catagen or early anagen phase, affecting the dermis (dermal papilla) and the tissue surrounding the hair follicle (dermal sheath), resulting in a smaller follicle and a reduced anagen phase (Figure 3A).
Completed clinical trials
Clincal proof-of-concept
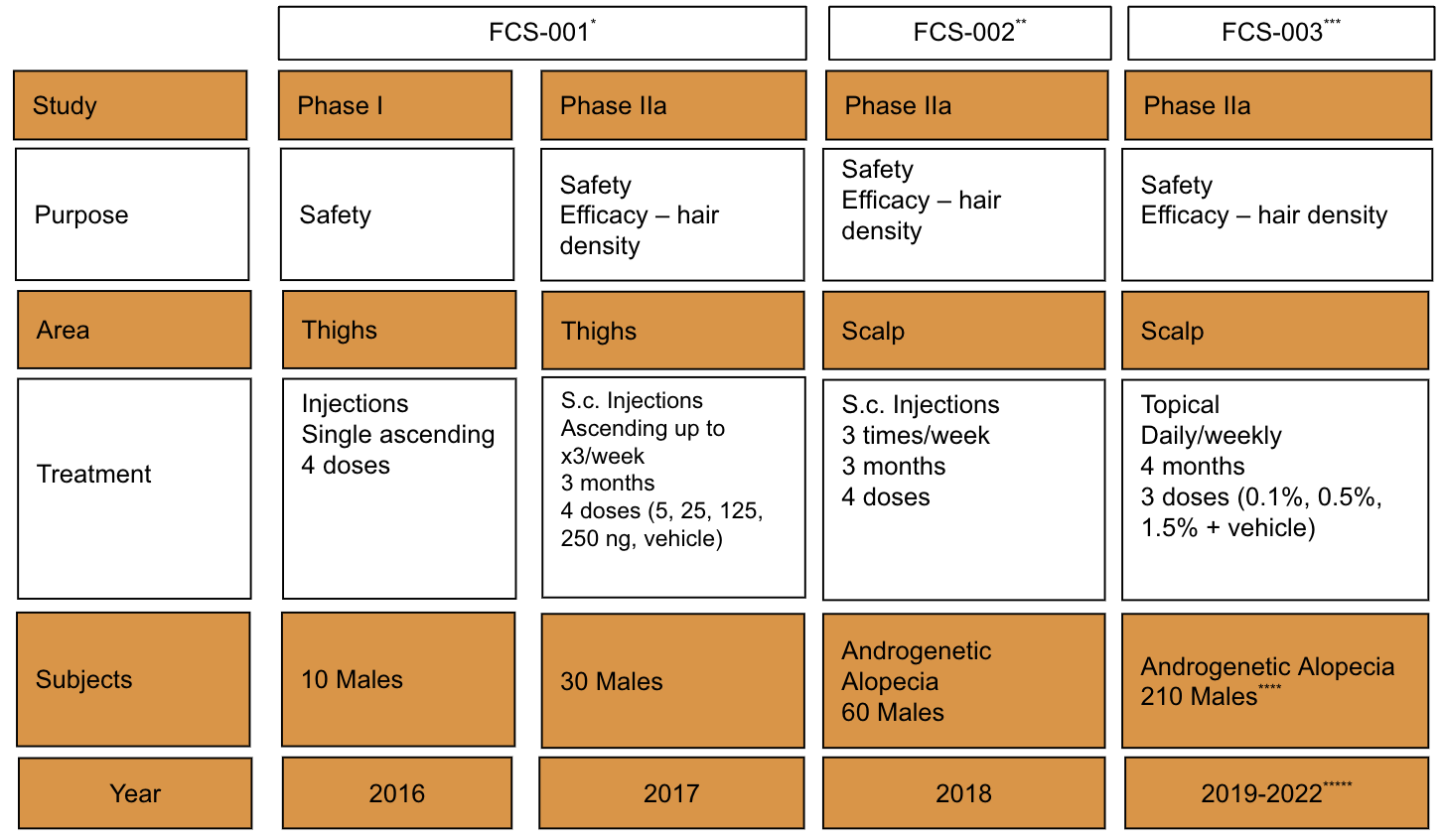
Three clinical trials have been conducted (FCS-001, FCS-002, and FCS-003), with subcutaneous (s.c.) and topical formulations (Table 2A). The FOL005 s.c. and topical formulations were both found to be safe and tolerable. All studies demonstrated that FOL005 stimulated growth of the new hairs.
*FCS-001: A randomized, double-blind, placebo-controlled phase I/IIa trial of FOL005 to investigate clinical safety and effect on hair growth in healthy volunteers
**FCS-002: A randomized, double-blind, placebo-controlled phase IIa trial of FOL005 to investigate efficacy on hair growth on scalp skin in alopecia subjects
***FCS-003: A randomized, double-blind, vehicle-controlled, dose-finding, multi-center, phase IIa trial of FOL005 topical formulations to investigate hair growth potential and safety in healthy male volunteers
****In total 199 patients were treated per protocol, of which 89 patients (45%) had a hair density below 255 hairs/cm2
*****Timeline impact due to COVID-19 pandemic
Latest completed clinical trials
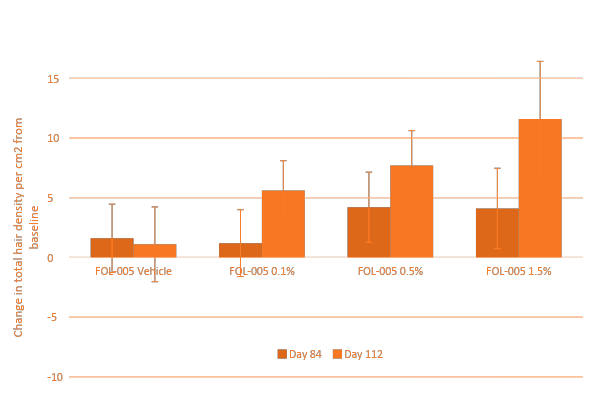
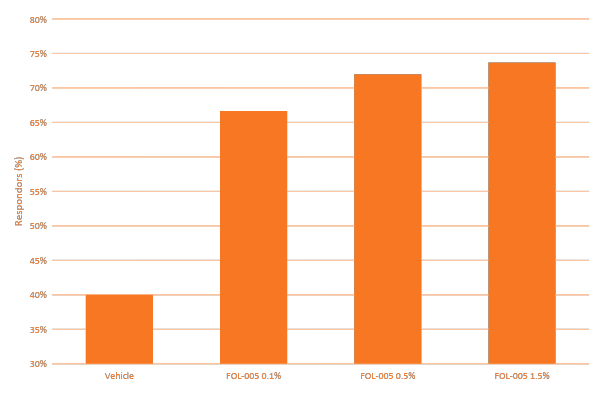
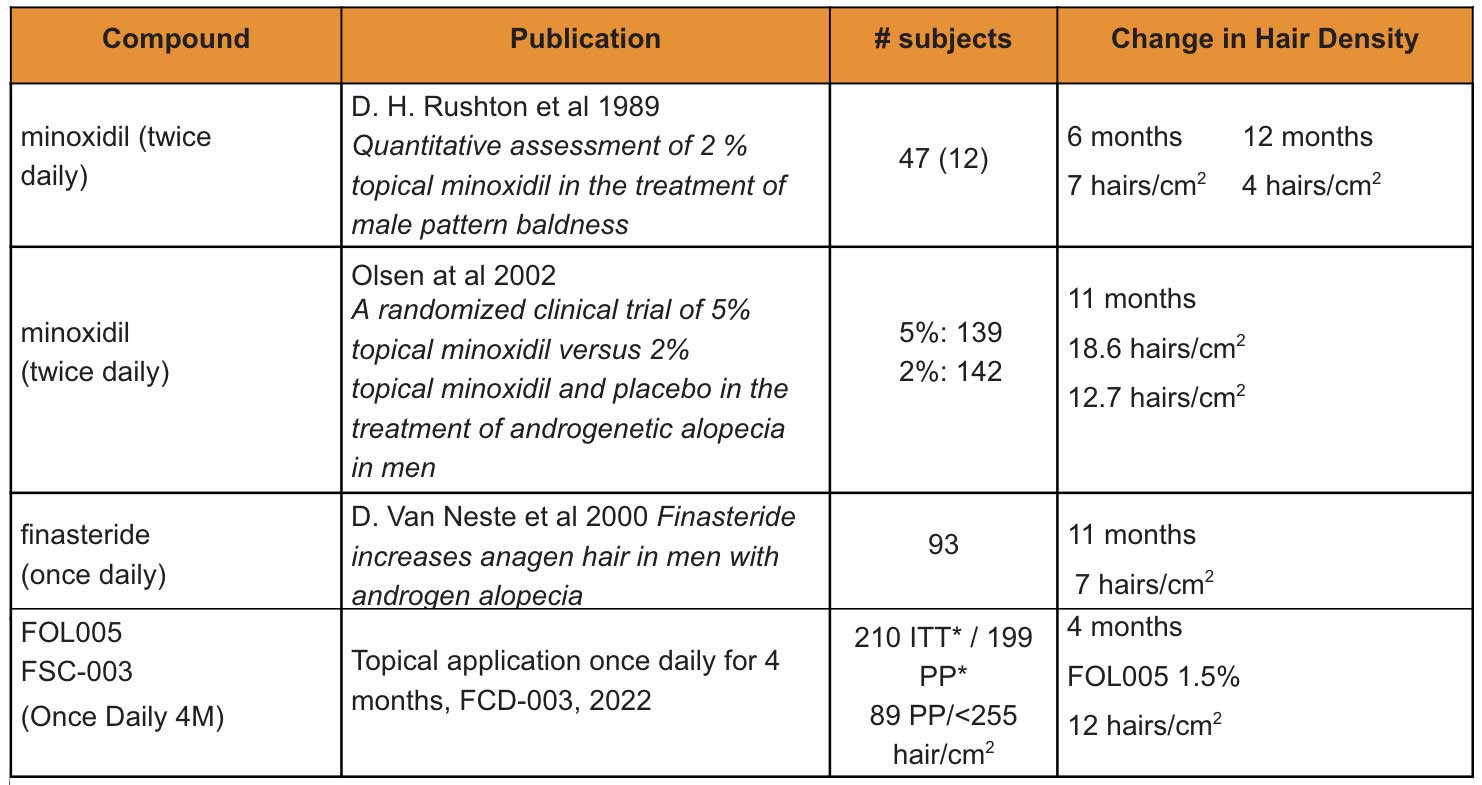
In the FCS-003 study a dose response effect was observed when applying FOL005 in those people with a hair density of less than 255 hair/cm2. Furthermore, FOL005 1.5% dose was on par with treatment effect reported for minoxidil and finasteride with a growth of 12 hairs/cm2 after only 4 months of use (Figure 7A), however with approximately 70% of the subjects responding to treatment (Figure 7B) compared to existing treatments where less than 40% responders effect is reported in the literature (Table 4).
Representative trichogram images
FOL005 1.5%
Difference in total hair counts 12 hairs/cm2 and non-vellus hair counts 12 hairs/cm2. Baseline counts 200 hairs/cm2 and Day 112 212 hairs/cm2.


FOL005 0.5%
Difference in total hair counts 7 hairs/cm2 and non-vellus hair counts 12 hairs/cm2. Baseline counts 252 hairs/cm2 and Day 112 259 hairs/cm2.


FOL005 0.1%
Difference in total hair counts 5 hairs/cm2 and non-vellus hair counts 13 hairs/cm2. Baseline counts 245 hairs/cm2 and Day 112 251 hairs/cm2.

FOL005 vehicle
Difference in total hair counts 0 hairs/cm2 and non-vellus hair counts -9 hairs/cm2. Baseline counts 223 hairs/cm2 and Day 112 223 hairs/cm2.


Superior hair growth stimulation
FOL005 has been shown to be safe and tolerable, and in the phase 2a clinical trial (FCS-003), the new product demonstrated high degree of hair growth stimulation with once daily topical application comparable to long term treatment with the competitors minoxidil and finasteride and with a much higher response rate (Table 4).
If we compare the results of the topical FOL005 study after just 4 months with ~12 hairs/cm2 in the <255 hairs/cm2 cohort*, the results are comparable with minoxidil studies after 11–12 months (see the table below).
Furthermore, FOL005 response rate is ~70%, compared with minoxidil with less than 40%.
Key benefits
- Efficacious and safe hair growth stimulator for men and women
- Equal or better efficacy than existing products on the market
- Higher number of responders than minoxidil and finasteride
- An non-running, easy to use topical formulation
- Less frequent administrations than minoxidil
Researchers and publications
Current and previous World Leading Scientific Advisors and Collaborators
FOL005 was developed by Professor Anna Hultgårdh Nilsson, Lund University in collaboration with LU Bioscience AB. Furthermore, Professor Jan Nilsson is active in progressing the mode-of-action of FOL005 at Lund University and in collaboration with Shanghai Changzheng Hospital, China. Furthermore, the topical formulation is very unique being able to ensure peptide delivery to the skin, recently published (Runnsjö, 2022). This work is supported by strong global patents in place and with protection to 2040. Over time several world leading scientists has been involved in the work with FOL005 (Table 3).

Professor Anna Hultgårdh Nilsson
Professor Anna Hultgårdh Nilsson Lund University. She holds a PhD in Medical Cell Biology from the Karolinska Institute. After a post doc position at Cedars-Sinai Medical Centre, University of California Los Angeles she returned to the Karolinska Institute where she studied the importance of the vascular smooth muscle cell in the onset of atherosclerosis. In 1998 Professor Hultgårdh Nilsson moved to Lund University and has continued to analyze cell and molecular mechanisms in the atherosclerotic process.

Dr Jan Nilsson

Professor Ralf Paus

Dr Maria Kasper

Professor Amos Gilhar

Dr Ulrike Blume-Peytavi

Dr Gerd Lindner